Quiz Summary
0 of 7 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
-

Daww you didn’t pass. Keep trying. You need to score at least 65% to pass.
-

Good! You scored above 65%. You have won a bronze medal. This earns you 1 braindollar! Go for gold?
-
Very good! You scored above 80%. You have won a silver medal. This earns you 10 braindollars! Go for gold?
-

Congrats! You scored above 90%! You have won a gold medal. This earns you 100 braindollars!
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 7
1. Question
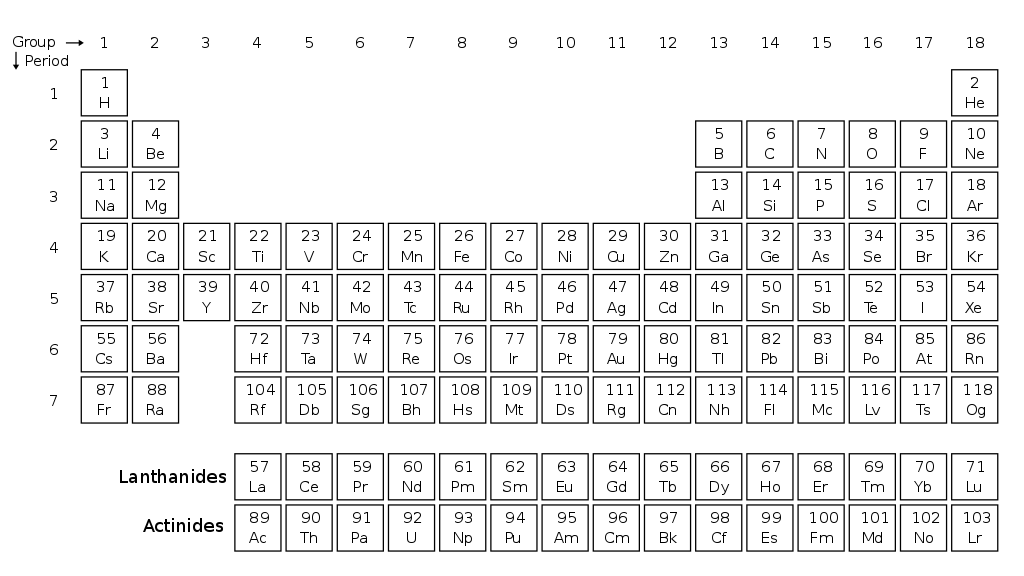
Which is the correct electronic configuration for titanium?
CorrectIncorrect -
Question 2 of 7
2. Question

Of the options provided below, which is the correct arrows-in-boxes diagram for carbon-14?
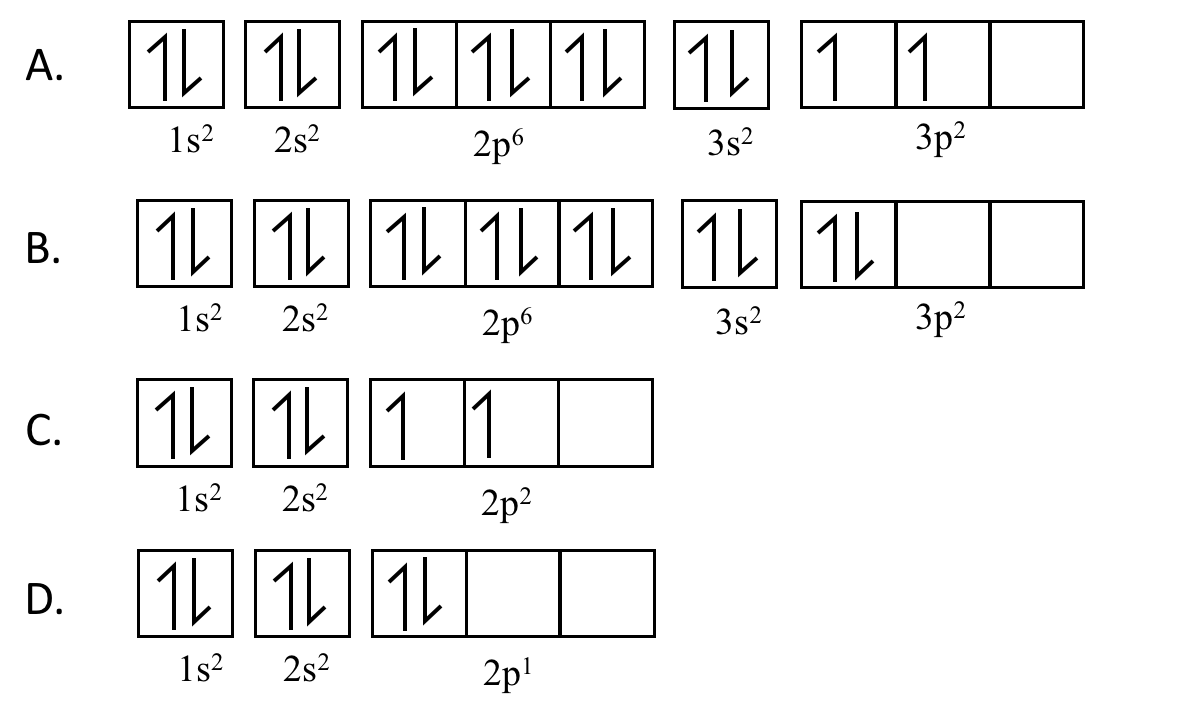 CorrectIncorrect
CorrectIncorrect -
Question 3 of 7
3. Question

Which is the condensed electron configuration for neutral copper?
CorrectIncorrect -
Question 4 of 7
4. Question

Which of these pairs of species have identical electronic configurations?
CorrectIncorrect -
Question 5 of 7
5. Question

Of the options below, which is the correct electronic configuration for chromium?
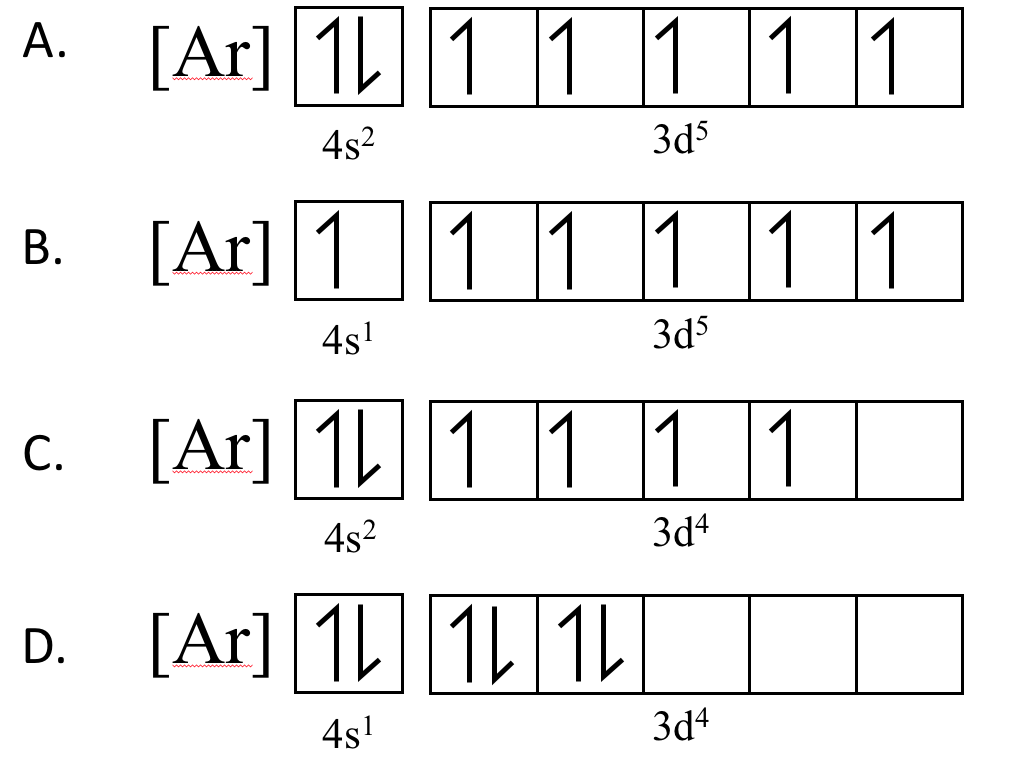 CorrectIncorrect
CorrectIncorrect -
Question 6 of 7
6. Question

Which is the correct electronic configuration for P3- ?
CorrectIncorrect -
Question 7 of 7
7. Question

The number of valence (highest-shell) electrons for an element can be deduced from its group in the periodic table. How many electrons are in the valence shell of oxygen?
CorrectIncorrect
