Quiz Summary
0 of 3 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
-

Daww you didn’t pass. Keep trying. You need to score at least 65% to pass.
-

Good! You scored above 65%. You have won a bronze medal. This earns you 1 braindollar! Go for gold?
-
Very good! You scored above 80%. You have won a silver medal. This earns you 10 braindollars! Go for gold?
-

Congrats! You scored above 90%! You have won a gold medal. This earns you 100 braindollars!
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 3
1. Question
.
-
A sample of iron has the following isotopic composition by mass.
54Fe: 6.4%
56Fe: 88.5%
57Fe: 5.1%
Calculate the relative atomic mass of iron based on these data.
Answer (to three sig figs):
CorrectIncorrect -
-
Question 2 of 3
2. Question
.
-
Naturally occurring silver has two stable isotopes, 107Ag and 109Ag. The relative atomic mass of silver is 107.87. Estimate the percentage abundance of 107Ag.
Answer (to three sig figs): %
CorrectIncorrect -
-
Question 3 of 3
3. Question
.
-
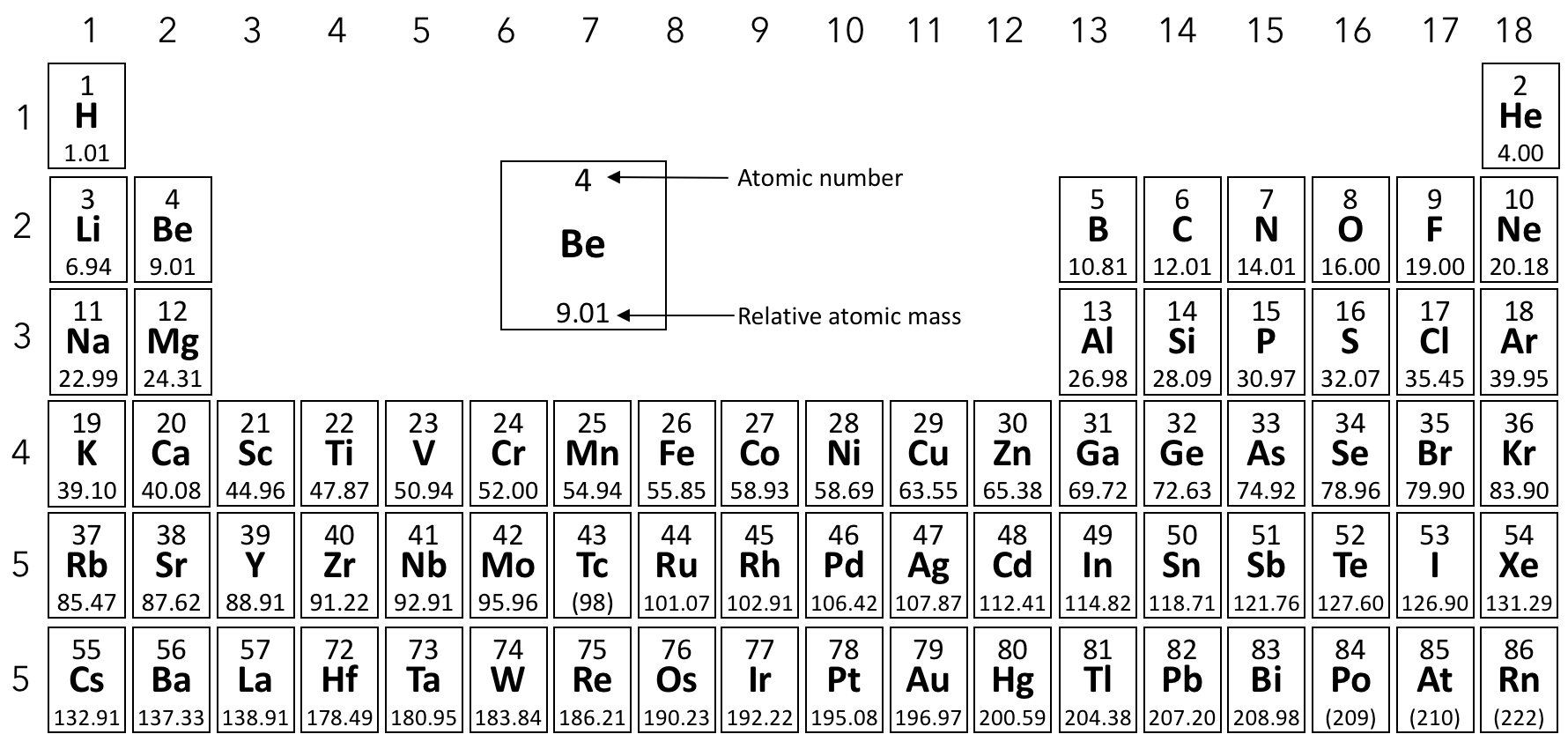
Magnesium has three stable isotopes, Mg-24, Mg-25 and Mg-26. The abundance of Mg-24 is 78.6%. Calculate the abundance of Mg-25 .
Mg-25 abundance (to three sig figs): %
CorrectIncorrect -
