Quiz Summary
0 of 4 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
-

Daww you didn’t pass. Keep trying.
-

Congrats, you scored above 70% You have won a bronze medal. This earns you 1 braindollar.
-
Congrats, you scored above 80% You have won a silver medal. This earns you 10 braindollars.
-

Congrats, you scored above 90% You have won a gold medal. This earns you 100 braindollars.
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 4
1. Question
Using the following image as a guide,
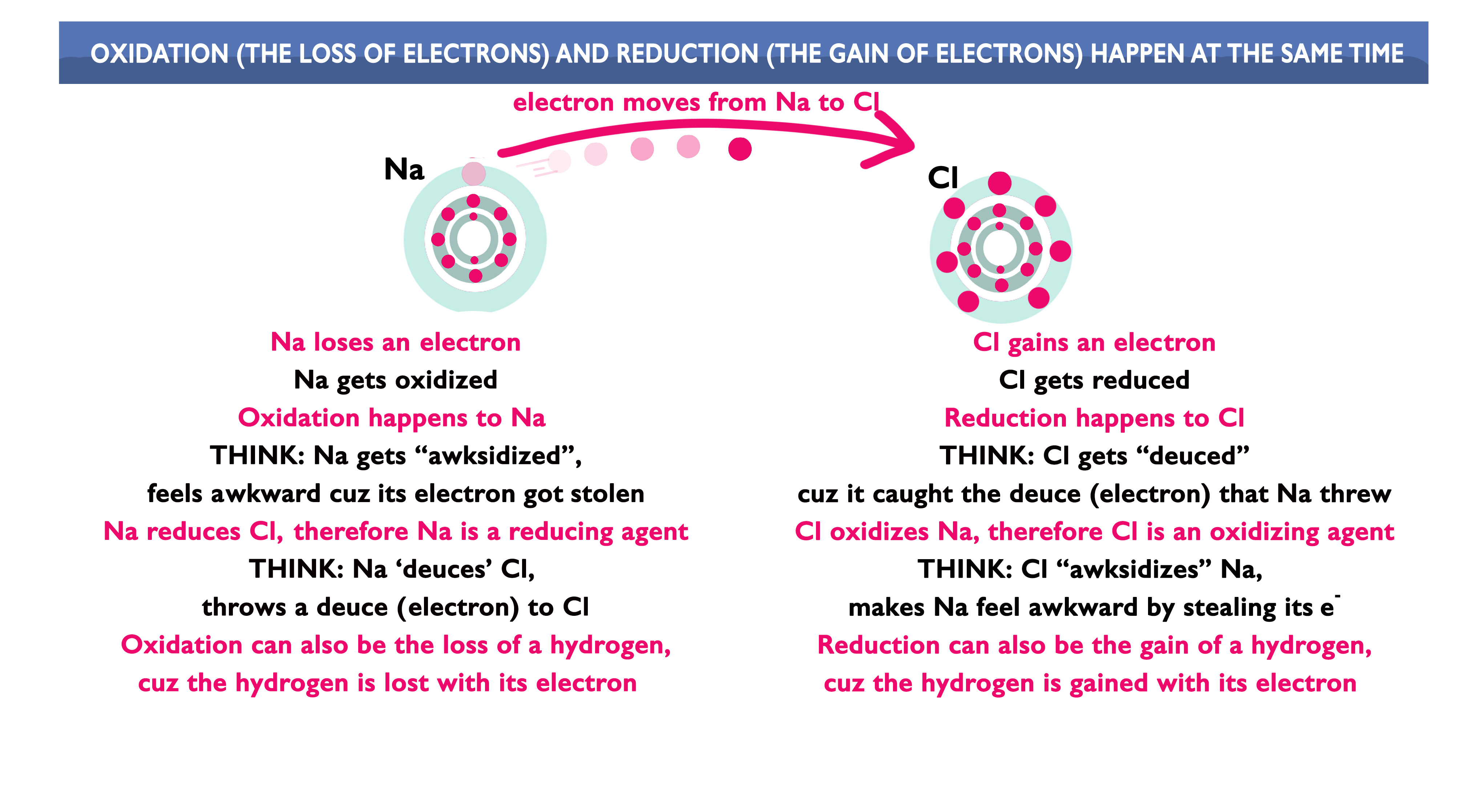
answer the questions below:
-
An element is easily oxidized if it has a high tendency to [ gain || lose ] electrons.
An element is easily reduced if it has a high tendency to [ gain || lose ] electrons.
CorrectIncorrect -
-
Question 2 of 4
2. Question
Using the following images as a guide,

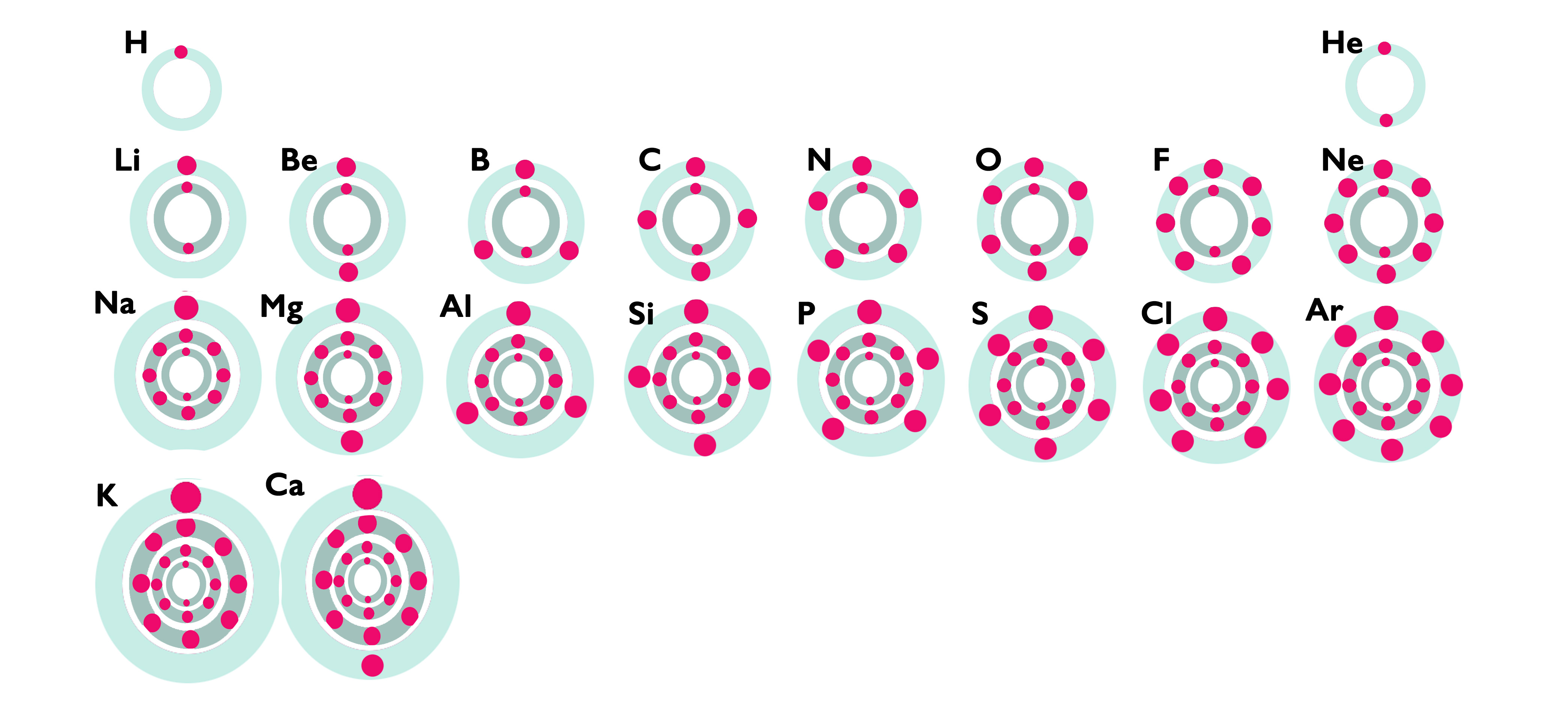
answer the questions below:
-
The most reactive metals, e.g. potassium, have a [ high || low ] tendency to donate electrons.
The most reactive non-metals, e.g. chlorine, have a [ high || low ] tendency to accept electrons.
CorrectIncorrect -
-
Question 3 of 4
3. Question
Using the following images as a guide,


answer the questions below:
-
The most reactive metals have a [ high || low ] tendency to oxidize other atoms.
The most reactive non-metals have a [ high || low ] tendency to reduce other atoms.
The most reactive metals have a [ high || low ] tendency to be oxidized by other atoms.
The most reactive non-metals have a [ high || low ] tendency to be reduced by other atoms.
CorrectIncorrect -
-
Question 4 of 4
4. Question
Using the following images as a guide,


answer the questions below:
-
To get Cl–, neutral chlorine must be [ oxidized || reduced ]
To get Na+, neutral sodium must be [ oxidized || reduced ]
To get O2-, neutral oxygen must be [ oxidized || reduced ]
Ca2+ is [ less || more ] oxidized than Ca+
CorrectIncorrect -
