Quiz Summary
0 of 3 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
-

Daww you didn’t pass. Keep trying.
-

Congrats, you scored above 70% You have won a bronze medal. This earns you 1 braindollar.
-
Congrats, you scored above 80% You have won a silver medal. This earns you 10 braindollars.
-

Congrats, you scored above 90% You have won a gold medal. This earns you 100 braindollars.
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 3
1. Question
Watch the following two videos and use them to answer the questions that follow
Sort elements
- loss
- gain
- Mg
- Ca
-
Oxidation is the _________ of electrons
-
Oxidation is the _________ of oxygen
-
In the reaction 2Mg + O2 → 2MgO. ___ is oxidized?
-
In the reaction 2Ca + O2 → 2CaO, ___ loses electrons?
CorrectIncorrect -
Question 2 of 3
2. Question
Watch the video (optional), then answer the questions that follow.
-
Compared to the ‘big boys of organic chemistry’ (carbon, nitrogen, oxygen and sulfur), hydrogen is at holding on to electrons. [ good || bad ]
If losing hydrogen is the same thing as losing an electron, oxidation can therefore be defined as the of hydrogen [ loss || gain ]
In the reaction 2H2S + 2O2 → S + 2H2O , sulfur is [ oxidized || reduced ] because it [ gains || loses ] hydrogen.
CorrectIncorrect -
-
Question 3 of 3
3. Question
This video will give you a way to remember the meanings of ‘oxidation’, ‘reduction’, ‘oxidizing agent’ and ‘reducing agent’. Use it to answer the questions below.
Here is the summary of the information from the video:
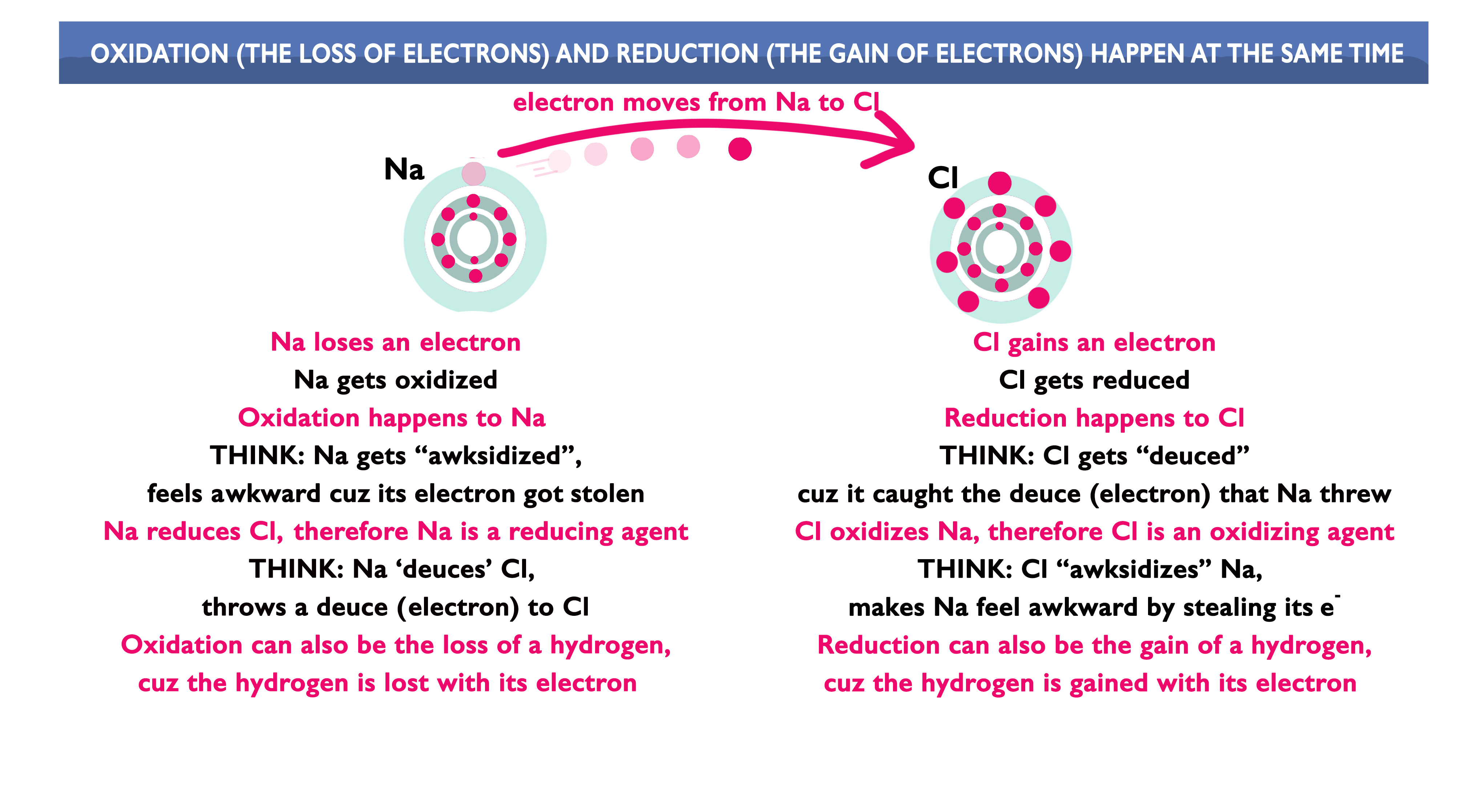
Now, the questions:
-
A way to remember oxidation is to think that when an element is oxidized, its electrons are stolen. It is therefore made to feel [ awkward || stupid ] , so it has been “awksidized”.
A way to remember reduction is to imagine that when an element is reduced, it is really ‘deuced’, so [ protons || electrons ] are thrown towards it. So we are thinking of an electron as a baseball.
A substance that is good at gaining electrons by stealing them from others is a good agent [ oxidizing || reducing ]. (Remember that when you steal electrons from other atoms you make them feel ‘awkward’.)
A substance that is good at donating electrons to others is a good agent [ oxidizing || reducing ] (Remember that when you donate, or ‘throw’, electrons to other atoms, you ‘deuce’ them.
CorrectIncorrect -
