Practice: Metallic properties
Quiz Summary
0 of 7 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 7 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
-
Good work! If you got 70% or above, you passed, and your quiz icon will change color. If you didn’t pass, keep trying until you do.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- Current
- Review / Skip
- Answered
- Correct
- Incorrect
-
Question 1 of 7
1. Question
.
-
The elements on the left of the periodic table are the , while the elements on the right of the periodic table are the [ non-metals || metals ]
CorrectIncorrect -
-
Question 2 of 7
2. Question
Metals have a tendency to______________, while non-metals have a tendency to ________________.
CorrectIncorrect -
Question 3 of 7
3. Question
-
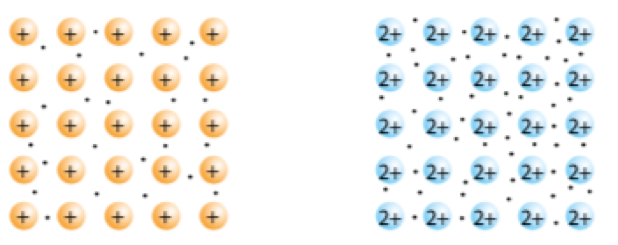
The picture above shows two metal lattices in a sea of free electrons. The orange metal is sodium, which only loses one electron. The blue metal is magnesium, which loses two electrons.
Of the two metals pictured, the [ orange || blue ] one has stronger electrostatic forces holding it together.
The [ orange || blue ] one would thus be expected to have a higher melting point.
CorrectIncorrect -
-
Question 4 of 7
4. Question
-
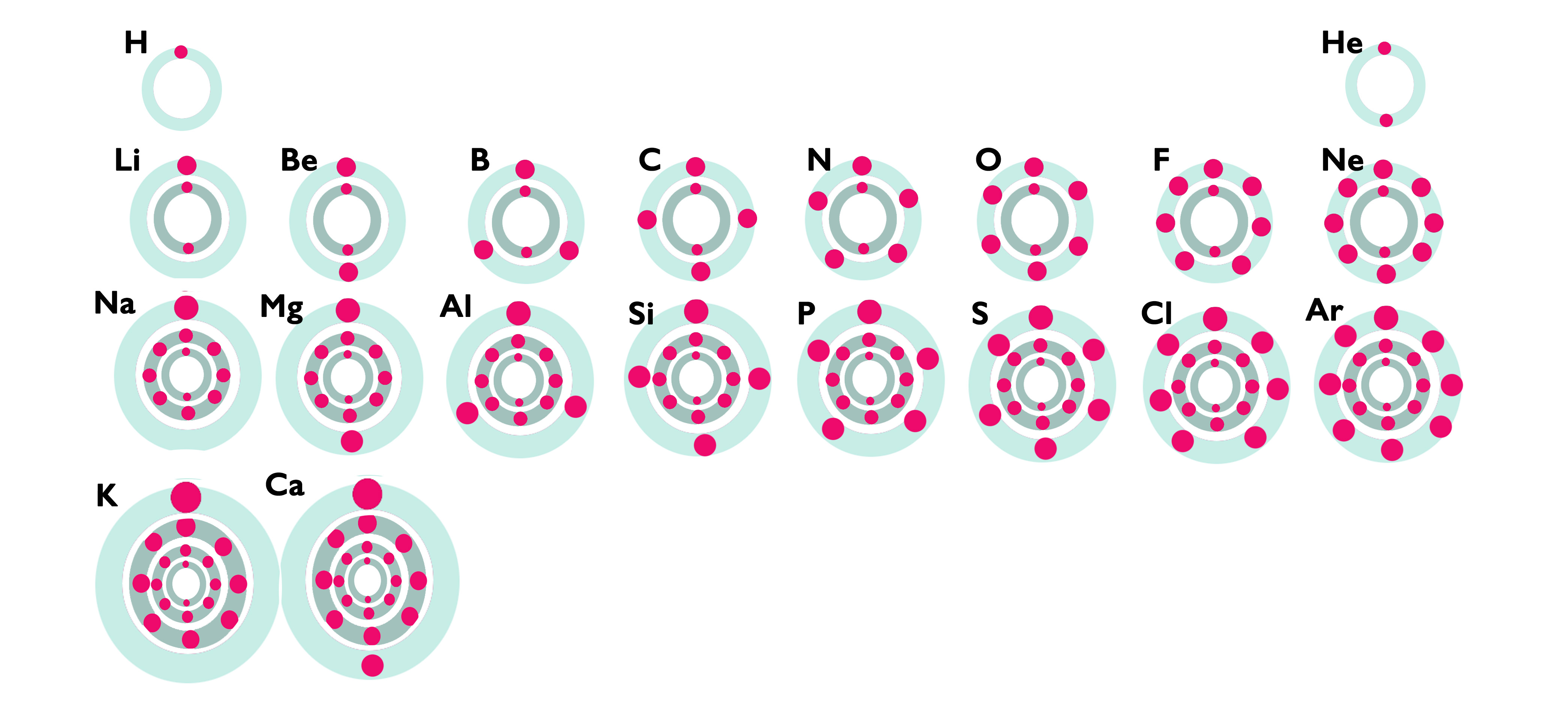
Arrange the period 3 metals in increasing order of their boiling point. You can type in symbols or full names.
Lowest boiling point: . Then: . Highest boiling point:
CorrectIncorrect -
-
Question 5 of 7
5. Question
.
-

Arrange the first 3 group 1 metals in increasing order of their reactivity. You can type in symbols or full names.
Lowest reactivity : . Then: . Highest reactivity:
CorrectIncorrect -
-
Question 6 of 7
6. Question
One of the metallic lattices below is sodium metal and the other is magnesium. Which is which?
CorrectIncorrect -
Question 7 of 7
7. Question
The blue atoms in the previous question are:
CorrectIncorrect
